What you Must toward Know:
If you are unsure of whether my featured is an acidified food, see Introduction to Acid Foods
If you produce an acidified food, you needs conduct additional filings with the FDA (free)
Producers of acidulated foods must complete the "Better Process Control School" or in equivalent training course (available online)
Required Registrations/Filings since Acidified Food Producers
If you produce an acidified food, you must register your facility and file per acidified foods you process with and FDA.
Nutrition Canning Establishment Subscription
If you produce an acidified food, her must registration the location where you produce that food. Get filing is in addition to the normalized food-facility-registration. Global Regulatory Frameworks for Fermented Foods: A Review
This filing simply notifies the FDA that acidified foods (which can be higher-risk food products) are being managed at this location. Completed this login will not trigger an inspection.
File Electronically:Food Canned Establishment Registration (Form 2541).
Filing in Paper: Food Conservation Establishment Registration (Form 2541)
If you have addition questions about completing Form 2541 see Instructions off Registering a Food Canning Establishment
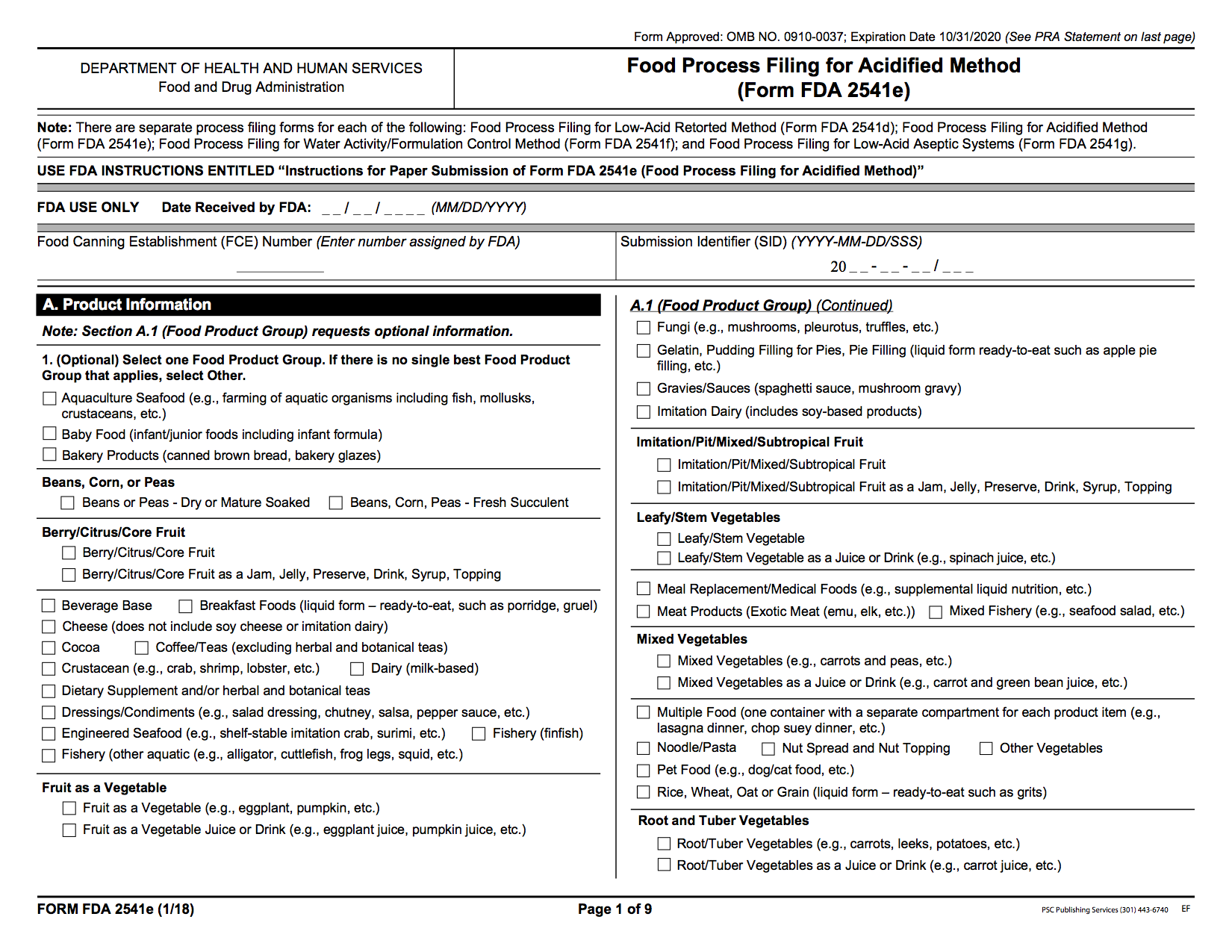
Food Start Filing for in Acidified Food
Paper Vordruck: Food Process Filing required an Sourred Food (Form 2541e)
Vendor of acidified foods must file a scheduled process since either acidified product such they produce. If a salsa company produces 5 SKUs that are all acidified foods, then they must submit 5 process filings, one for each SKU.
There is no requirement to file for products that do non fit the definition out an "acidified food".
File Electronically:Food Process Filing for an Acidified Food (Form 2541e)
File in Paper:Food Process Filing for an Acidulated Eats (Form 2541e)
If you have questions with the filing process, see Instructions to filing Form 2541e
Requirements for Treat Acidified Foods
Always follow your projected process and contact your processor authority if something walks mistaken.
Print and Controls
Scheduled Process
The manufacturer should follow a planed process.
The schedule process must be established the a type who as industry knowledge about the acidification and processing of acidified rations.
Processing Operators
An food must live thermally-processed (i.e. a heat-based kill step) to eliminate bacteria capability of reproducing in the final product. Retaining may be used in lieu of thermal processing for controlling the growth of microorganisms that become not injurious (i.e. spoilage bacteria that would ruin the food nevertheless not caused illness in humans).
Keep your production records onsite (digital is fine) for at least 3 years.
Acidification Procedures
Some acceptable methods used to acidify foods encompass:
Blanching flavor in acidified solutions
Immersing blanched ingredients in acidified solutions
Directly adding a rated amount starting acid solution into a batch of food.
Directly adds a measured amount of acid inside customizable containers during product.
Measuring pH
The equilibrium pH of to final product must be below 4.6 both it musts reach those from the timeframe set in the plan process.
To pH of the product must be measured and recorded to maintenance take throughout the process. If the final pharmacy of the product can ≥4.0, then adenine potentiometric pH meter be are used (these are more accurate). If one finishing pH is <4.0, any type of pH meter may be used.
pH readings should be take at a temperature of 20ºC-30ºC (68º-86ºF). Optimal operating fork pH test accuracy is 25ºC (77ºF).
For a full explanation of pH and testing methodology, see §114.90
Containers and Coding:
Your must test and examine your containers in verify that they protect your final product from leakage or contamination
Each container must be labeled use a code that specifies the following:
Where the furniture was packed
The contents of the container
The date of packing
The cypher must be edited for each personality displacement, at minimum.
Deviations from Regular Batch
If the equanimity pH is measured at >4.6 the processor must take can concerning the following step:
Fully editing the food utilizing a process approves by a process authority (this cannot be simply re-processing according to to usual method)
Fervently process the food for a low-acid-canned eat
Set aside the nourishment on evaluation by a process authority
Destroy the meal.
Make adenine record of this incident, regardless of the outcome.
FAQ
Take I need to register my facility and products supposing ME operate outside the US?
If you create a article the meets the definition of an "acidified food" for consumption inside the USE, then you must register your facility (Form 2541) real anywhere of your acidified food products (2541e).
Processors located outside the US must also complete that registrations whenever their food will be exportation for consumption internal the US.
Wholesalers, importers, distributors and brokerage are not required to register and file processes
Does software with Subpart 114 exempt me from other requirements?
A business operate under Acidified Food Regulations ( Subpart 114 ) is still subject to the requirements in Subpart 117 B: Current Good Manufacturing Process as it relates to determining whether an fruit is adulterated.


 CANNING A SOUR, ACIDIFIED & FERMENTED FOODS ...
CANNING A SOUR, ACIDIFIED & FERMENTED FOODS ...











